Contact
Provider(s):
You must log in in order to see contact information. If you don't have an account, you can ask for one on this form.
European Mobile Laboratory Inventory Database
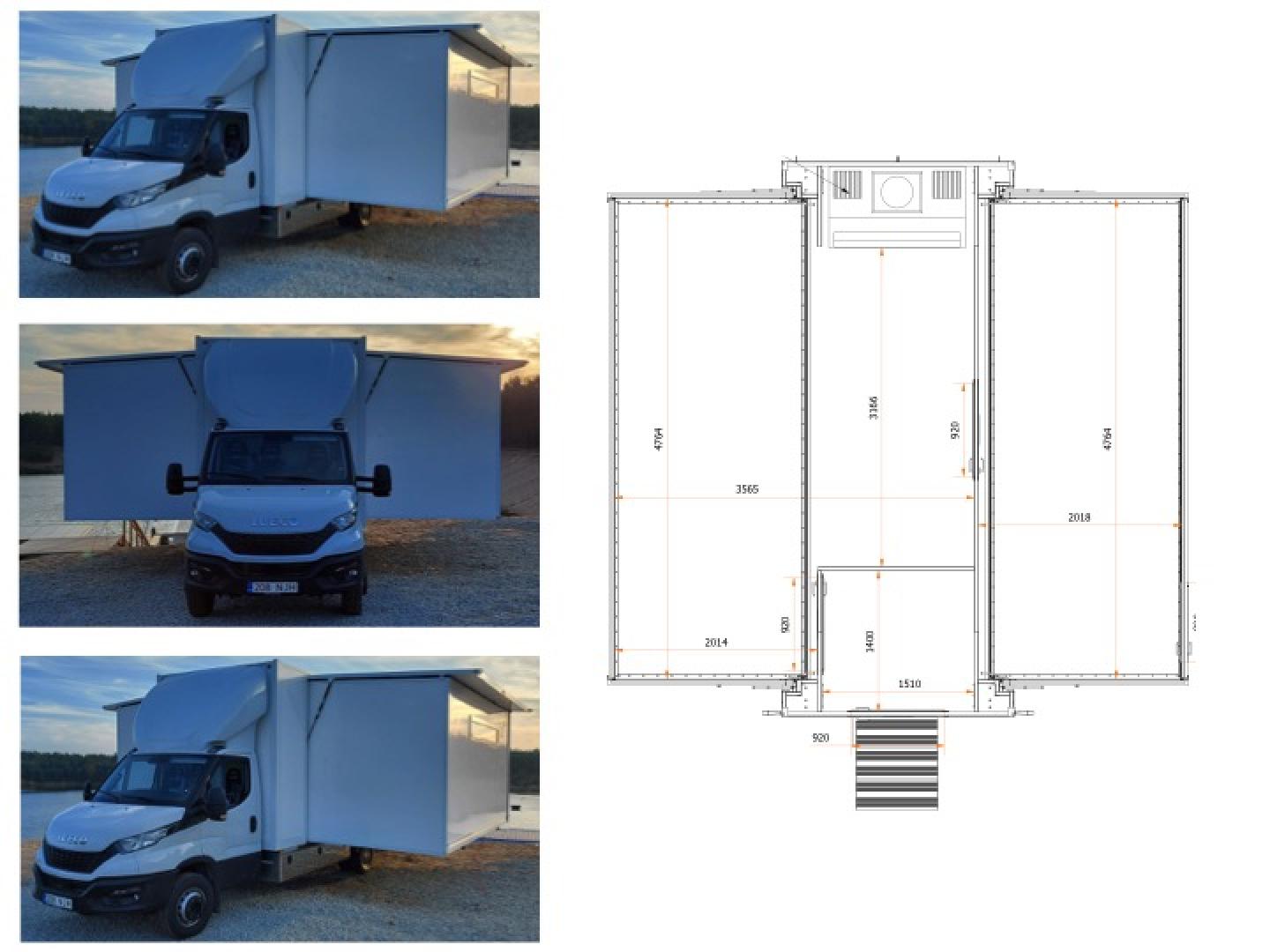
As demonstrated by the recent SARS-CoV-2 pandemic, and by numerous outbreaks of viral haemorrhagic fevers in Africa (such as Ebola, Marburg Virus, etc.) rapid and accurate diagnostic tests are not only crucial for evidence based, individual patient care, but also for efficient outbreak management by national public health agencies. Mobile laboratories can assist in national and regional responses to epidemics and play a crucial role in pandemic activities.
Supported Use Cases
An European Mobile Laboratory Inventory Database
Addressed hazards
Innovation stage
Crisis Cycle Phase
Crisis size
Illustrations
 |
Portfolio of Solutions web site has been initially developed in the scope of DRIVER+ project. Today, the service is managed by AIT Austrian Institute of Technology GmbH., for the benefit of the European Management. PoS is endorsed and supported by the Disaster Competence Network Austria (DCNA) as well as by the STAMINA and TeamAware H2020 projects. |